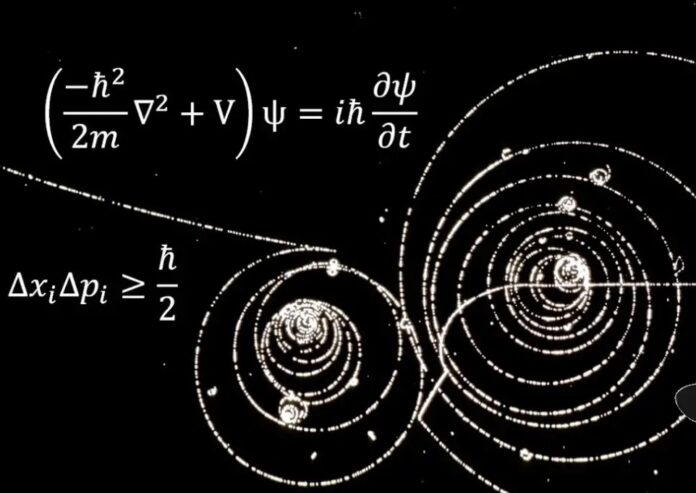
The Creation of the Heisenberg Uncertainty Principle
The Heisenberg Uncertainty Principle is a principle in quantum mechanics that states that the more precisely the position of some particle is determined, the less precisely its momentum can be known and vice versa. It was first published in 1927 by German physicist Werner Heisenberg. The Heisenberg Uncertainty Principle is committed to the following statements:If either one of time or space is measured, then the position and momentum of a particle cannot be accurately known. If one does not know the position and momentum, then it is impossible to predict future events. For example, if one could measure both time and space precisely with a clock-like device like a stopwatch, then it would be possible to calculate all events at that moment. If time and space are not known precisely, then an event cannot be calculated.The Heisenberg Uncertainty Principle is committed to the following statements: If either one of time or space is measured, then the position and momentum of a particle cannot be accurately known.If one does not know the position and momentum, then it is impossible to predict future events. For example, if one could measure both time and space precisely with a clock-like device like a stopwatch, then it would be possible to measure the size of a black hole in terms of time and space. If someone had a device that could measure time, it would be possible for them to show how often light emitted by stars is blocked by Jupiter’s shadow during one orbit.
The History and Development of the Uncertainty Principle
The uncertainty principle is one of the most important principles in quantum mechanics. It states that the more precisely a particle’s position is determined, the less precisely its momentum can be known, and vice versa.
The uncertainty principle was first postulated in 1927 by Werner Heisenberg to describe a certain property of quantum objects. Heisenberg’s original interpretation was as a generalization of Max Planck‘s energy law from 1900 which states that the energy E for an electromagnetic wave depends on frequency f according to
E=hf
This leads to an uncertainty in energy of ΔE∼h/f. In this way, it can be seen as a consequence of quantum theory itself (as opposed to any limitations on measurement).
The Heisenberg Uncertainty Principle’s Limits and Benefits
The Heisenberg Uncertainty Principle is a principle in quantum mechanics that states that there are limits to what we can know about the position and momentum of a particle.
This principle also has benefits. For example, it can be used to measure how much energy an electron has. It also helps us understand how atoms behave and how they form molecules. Energy has both a unit and a typeIt is important to know how much energy can be measured and what type it is. For example, nuclear power plants use an amount of energy that is measured in watts, but the level of radiation emitted from those plants varies based on the type of radiation used.
How It Impacts Your Daily Life and What You Need to Know about it
This principle has an impact on our daily lives as we are constantly bombarded with digital information. It is impossible to know which information is accurate and which is fake.
The Heisenberg uncertainty principle, also known as Heisenberg’s Uncertainty Principle, states that it’s impossible to know both the position and momentum of a particle at any given time. This means that we can’t be sure if what we’re seeing or hearing is true or not, because there are too many uncertainties involved- hence why this principle has an impact on our daily lives as we are constantly bombarded with digital information.